Technical expertise driving excellence.
DPI respiratory profile analysis and our recommendations
Context
Dry powder inhalers (DPIs) are commonly used for administering asthma and chronic obstructive pulmonary disease (COPD) treatments. DPIs are popular with healthcare providers because they deliver the medication directly to the lungs, avoiding issues associated with Metered Dose Inhalers. (MDI).
DPI has the following main advantages over MDI :
- No propellants: DPIs do not use gas propellants such as chlorofluorocarbons (CFCs) or hydrofluoroalkanes (HFAs), that are found in MDIs. This makes DPIs more environmentally friendly and reduces the environmental concerns associated with the use of propellants.
- Ease of use: DPIs are often considered easier to use as they do not require the user to coordinate inhalation with the actuation of the device. With a DPI, patients simply inhale through the device to trigger the release of the medication.

- Drug stability: Medicines in dry powder form are generally more stable and have a longer shelf life than the suspension formulations used in MDIs. This can reduce the need for preservatives and other additives.
- Less dependence on inhalation technique: DPIs are less dependent on the patient’s inhalation technique. With an MDI, poor co-ordination or inhaling too quickly can result in inadequate drug deposition in the lungs. DPIs require strong, deep inspiration, which patients often find easier to control.
- Cost: In some cases, DPIs can be more cost-effective in the long term as they do not require expensive propellants and have a longer shelf life.
The benchmarks in this area are the recommendations of the United State Pharmacopeia (USP Chapter 601) and the European Pharmacopeia (EP Chapter 0671) [1]. Standardized testing methods eliminate numerous biases and enable reliable comparison of results.
These standardized methods quantify the dose of powder extracted from the device (Delivered Dose Uniformity) or the size and distribution of the particles (Aerodynamic Particle Size Distribution).
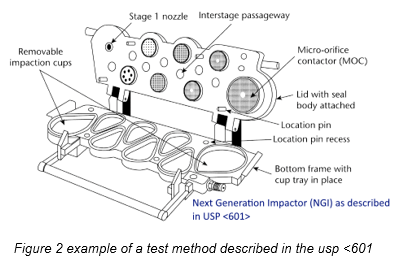
In both cases, the formulation is extracted using a defined vacuum profile: products are subject to a rectangular distribution of negative pressure, starting at 0 kPa and instantly rising to 4 kPa, ending once 4 liters have been drawn in. At first glance, this seems like a reasonable breathing pattern for an average adult.
However, a closer analysis of this respiratory profile through the prism of time, depression and volume reveals significant limitations in relation to the actual DPI user.
Analysis of Food and Drug Administration (FDA) / European Medicine Agency (EMA) standard respiratory profile
4 kPa vacuum: achievable by all patients?
Most hospital studies [2] [3] suggest that an order of magnitude of 4 kPa seems appropriate. However, this value should be considered a “peak” value (ranging from 4 kPa to 6 kPa), which differs from the constant value adopted by regulatory bodies.
Though the data is limited, it suggests that the maximum vacuum value may be proportional to product resistance. This is a crucial point when analyzing low-resistance devices, because they may prevent the user from reaching 4 kPa. Additionally, while this value is within the capabilities of an adult with COPD or asthma, it seems difficult to achieve for an asthmatic child. This is a factor that should not be overlooked when developing a DPI for children.
4-litre volume: inspirable for a sick user?
Data from the large Global Lung Initiative 2012 study [4], used for diagnosis with a spirometer, and World Health Organization growth data show that 4 litres is only representative of an average healthy male. The target population for DPIs, because of their age or sex and the severity of their pathologies, cannot necessarily reach this volume.
Alternative values could be proposed depending on the DPI target tested :
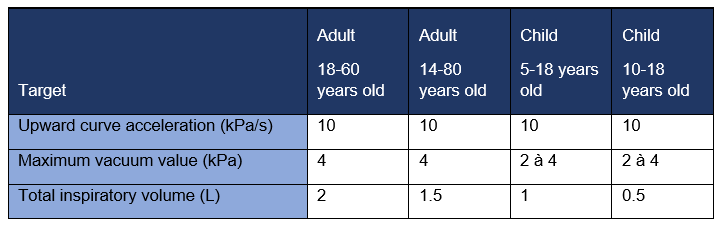
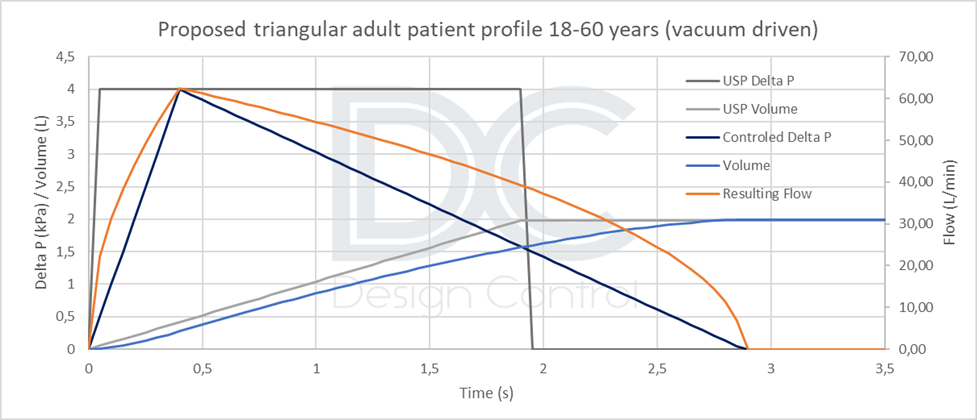
- Approximately 2 liters for an adult population [18 to 60 years], or more conservatively 1.5 liters for a larger adult population [14 to 80 years].
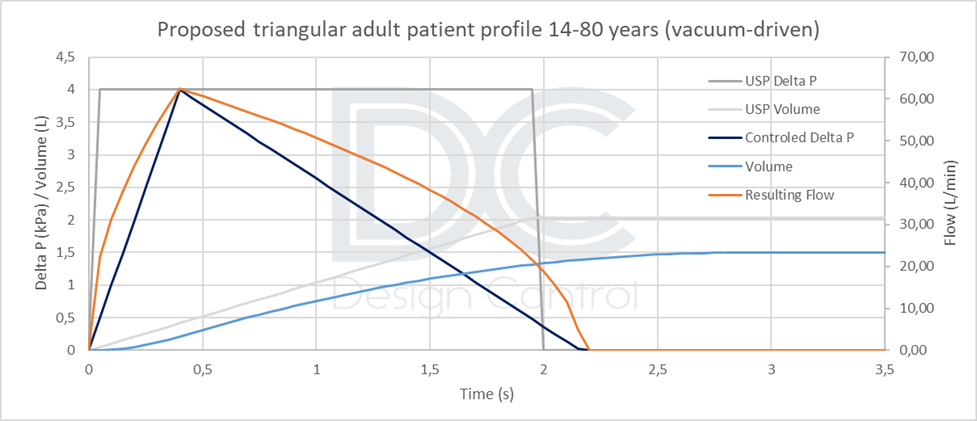
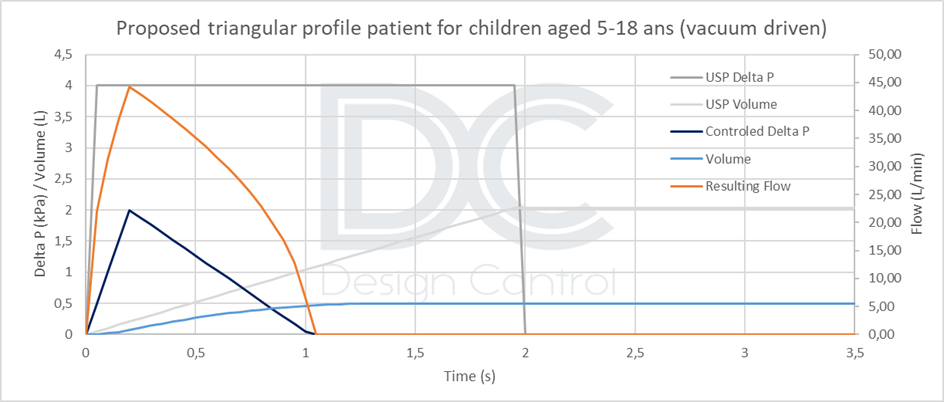
- A lower value of 0.5 L for children aged [5 to 18], or 1 L for a limited population aged [10 to 18].
This value of 2L is also adopted in the FDA guidance on DPI/MDI [5] and in the USP 601, as revised in 2018 [6].
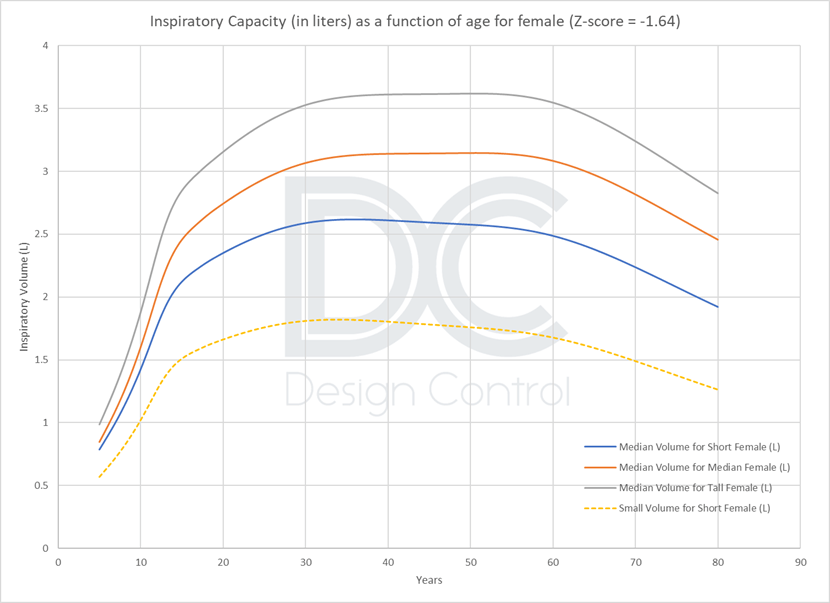
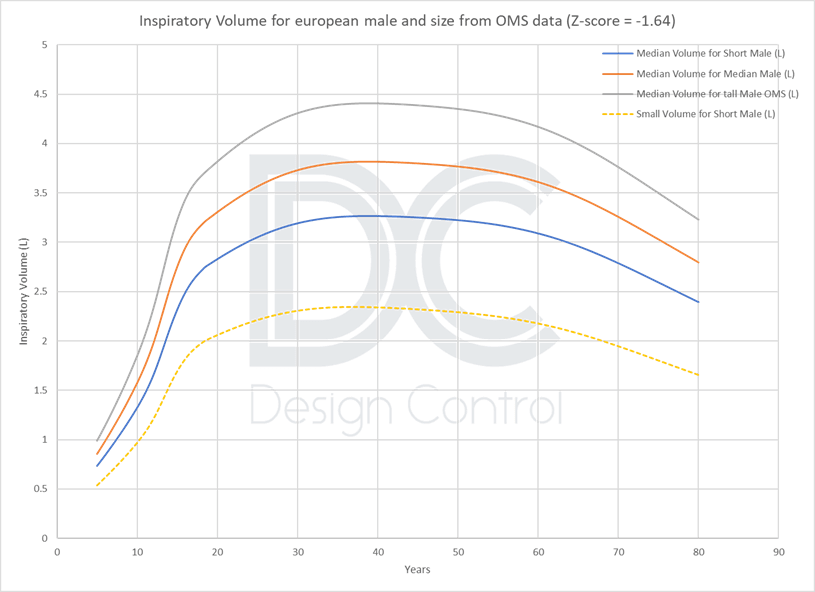
Rectangular respiratory profile: Representative of a patient profile?
Clearly, the answer is no. All breath profiles, whether from a healthy or diseased person, have a flattened bell shape. The rectangular profile is merely a technical convenience that corresponds to the abrupt opening and closing of a valve.
Unfortunately, using this profile biases the analysis of the DPI’s performance. Creating a sudden vacuum (an instantaneous change from 0 to 4 kPa) favors the extraction and decompaction of the powder. This can lead to an overestimation of the product’s actual performance. This gives certain products an unfair advantage over others and can result in the validation of technical solutions that do not align with patients’ actual respiratory profiles.
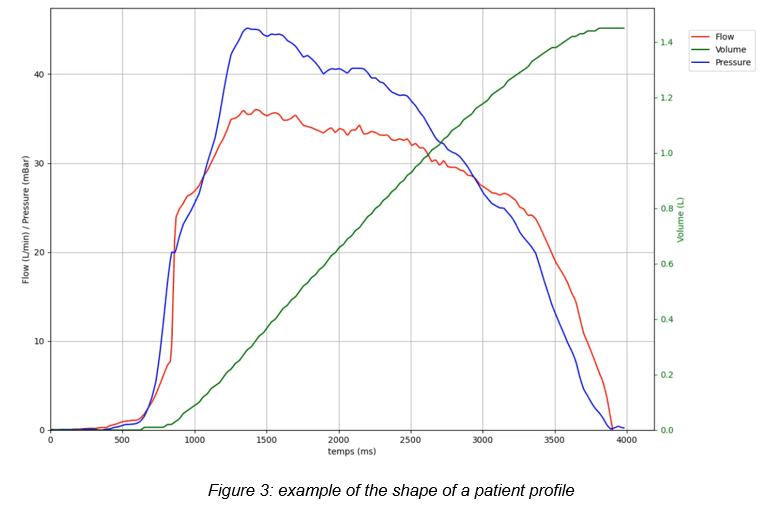
An analysis of various profiles in the literature shows that patients can generate a vacuum with an acceleration of about 10 kPa/s. For instance, this would entail going from 0 to 4 kPa in 0.4 seconds. Therefore, we are far from instantaneous depression.
Conclusion and proposed respiratory profiles for the target population.
When testing a DPI-type device under conditions similar to those encountered in vivo, the rectangular patient profiles recommended by the main regulatory agency are no longer suitable. These profiles often cause engineers to favor certain solutions and overestimate the performance of products in terms of yield, decompaction, and therapeutic efficacy.
Therefore, unless a real patient profile can be simulated (standardized, reproducible, and representative), the triangular vacuum profile appears to be a good compromise. Implementing this profile is not much more technically difficult than implementing a rectangular profile, and it will be more conservative than a real profile. The maximum negative pressure and inspired volume values must be adapted according to the target population. Profiles are calculated according to the product’s resistance.
At Design Control Consulting, we systematically integrate these profiles and the recommendations of regulatory agency to guarantee the relevance of our performance analyses and the reliability of our DPI solutions.
Bibliography
| [1] | «European Pharmacopoeia» [Online]. Available: https://pheur.edqm.eu/home. |
| [2] | C. P. H. H. S. D. S. J. C. H. Azouz W, «The inhalation characteristics of patients when they use different dry powder inhalers » Aerosol Med Pulm Drug Deliv., n° %1doi: 10.1089/jamp.2013.1119. Epub 2014 May 9. PMID: 24815999., pp. 35-42, 2015. |
| [3] | W. J. D. R. Clark AR, «The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates.,» J Aerosol Med Pulm Drug Deliv, n° %1doi: 10.1089/jamp.2019.1556. Epub 2019 Oct 31. PMID: 31613682; PMCID: PMC7041319., pp. 1-11, 2020. |
| [4] | S. S. T. J. C. Philip H. Quanjer, «Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations,» [Online]. Available: https://publications.ersnet.org/content/erj/40/6/1324.full.pdf. |
| [5] | F. &. D. Administration, “Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products – Quality Considerations,” [Online]. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/metered-dose-inhaler-mdi-and-dry-powder-inhaler-dpi-drug-products-quality-considerations. |
| [6] | «USP <601> INHALATION AND NASAL DRUG PRODUCTS: AEROSOLS, SPRAYS, AND POWDERS – PERFORMANCE QUALITY TESTS». |
Author : F. Moïa





